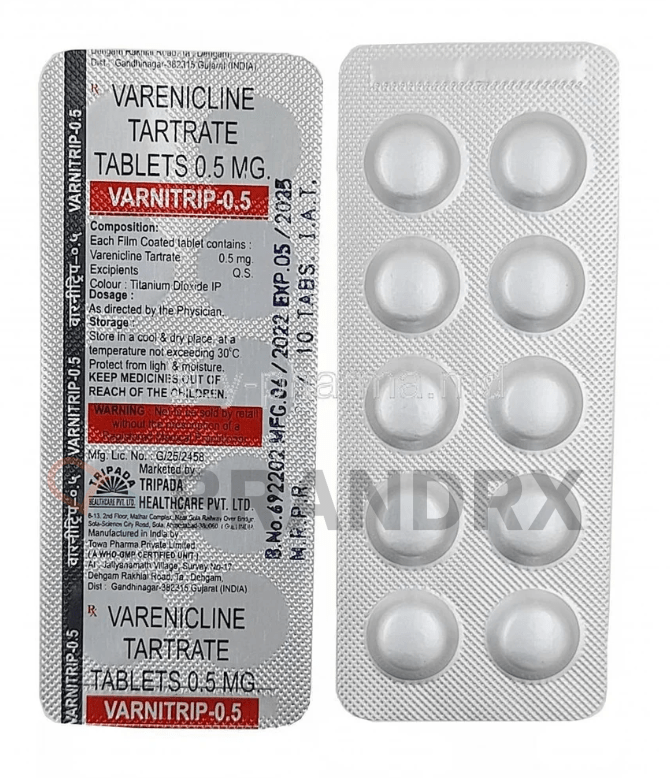
| Active substance | Varenicline |
|---|---|
| Chemical name | (7aS)-7,8,9,10-tetrahydro-6,10-methano-6H-pyrazino[2,3-h][3]benzazepine |
| Side effects | Nausea, headache, vomiting, flatulence, insomnia, abnormal dreams, and changes in taste |
| Effects | Reduces craving and withdrawal symptoms associated with quitting smoking |
| Dosage (sports) | Not applicable |
| Dosage (medical) | Typically starts with 0.5 mg once daily for the first 3 days, then 0.5 mg twice daily for the next 4 days, then 1 mg twice daily thereafter |
| Half-life | Approximately 24 hours |
| Main action | Smoking cessation aid |
| Substance class | Partial nicotinic acetylcholine receptor agonist |
| Formula | C13H13N3 |
| Storage conditions | Store at room temperature away from moisture and heat |
| Trade name | Chantix, Champix |
| Blood pressure | Generally has no significant effect on blood pressure |
| Also known as | Champix |
| Strength | 0.5mg |
| Lab Test | Not typically monitored with lab tests |
| Hepatotoxicity | Minimal to none |
| Water Retention | No significant water retention |
| Use in sports | Not typically used in sports |
| Manufacturer | Tripada Healthcare Pvt. Ltd. |
| Packing | 10 tabs/blister |





There are no reviews yet.